Summary
AIMS OF THE STUDY
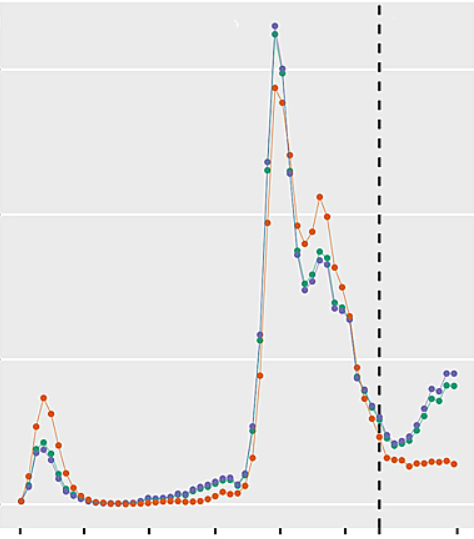
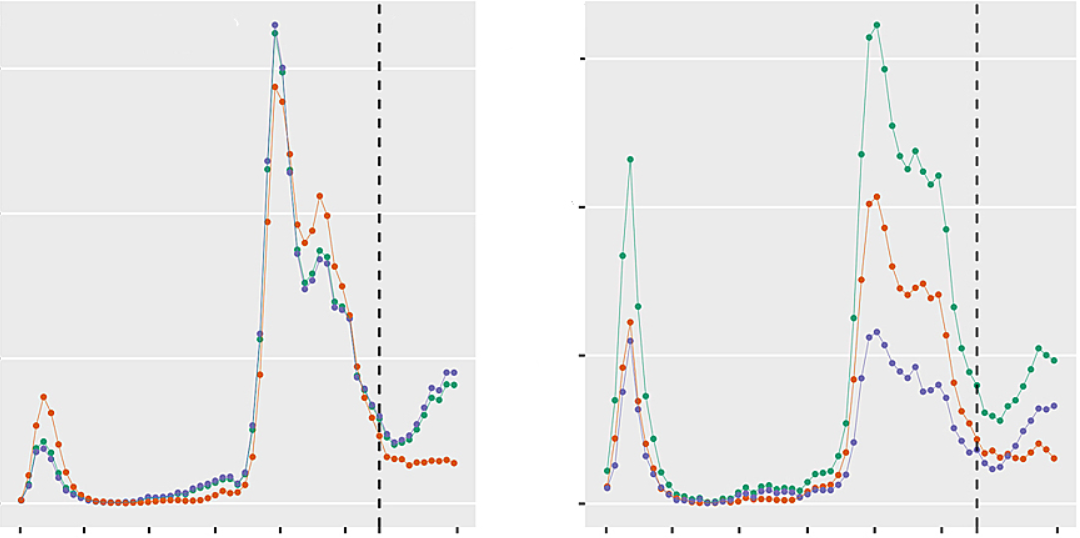
During the ongoing COVID-19 pandemic, the launch of a large-scale vaccination campaign and virus mutations have hinted at possible changes in transmissibility and the virulence affecting disease progression up to critical illness, and carry potential for future vaccination failure. To monitor disease development over time with respect to critically ill COVID-19 patients, we report near real-time prospective observational data from the RISC-19-ICU registry that indicate changed characteristics of critically ill patients admitted to Swiss intensive care units (ICUs) at the onset of a third pandemic wave.
METHODS
1829 of 3344 critically ill COVID-19 patients enrolled in the international RISC-19-ICU registry as of 31 May 2021 were treated in Switzerland and were included in the present study. Of these, 1690 patients were admitted to the ICU before 1 February 2021 and were compared with 139 patients admitted during the emerging third pandemic wave
RESULTS
Third wave patients were a mean of 5.2 years (95% confidence interval [CI] 3.2–7.1) younger (median 66.0 years, interquartile range [IQR] 57.0–73.0 vs 62.0 years, IQR 54.5–68.0; p <0.0001) and had a higher body mass index than patients admitted in the previous pandemic period. They presented with lower SAPS II and APACHE II scores, less need for circulatory support and lower white blood cell counts at ICU admission. P/F ratio was similar, but a 14% increase in ventilatory ratio was observed over time (p = 0.03)
CONCLUSION
Near real-time registry data show that the latest COVID-19 patients admitted to ICUs in Switzerland at the onset of the third wave were on average 5 years younger, had a higher body mass index, and presented with lower physiological risk scores but a trend towards more severe lung failure. These differences may primarily be related to the ongoing nationwide vaccination campaign, but the possibility that changes in virus-host interactions may be a co-factor in the age shift and change in disease characteristics is cause for concern, and should be taken into account in the public health and vaccination strategy during the ongoing pandemic. (ClinicalTrials.gov Identifier: NCT04357275)
Introduction
The coronavirus disease (COVID)-19 pandemic, declared on 17 March 2020 by the World Health Organization (WHO), has burdened the global health systems without recent precedent. A rapid international effort in uncovering properties of the novel disease and optimal treatment strategies was followed by the launch of a large-scale vaccination campaign . At the same time, virus mutations have hinted at possible changes in (i) transmissibility and (ii) the virulence affecting disease progression up to critical illness and mortality , and carry the potential for future vaccination failure. Over the last 12 months, a renewed increase in SARS-CoV-2 cases in Switzerland and reported changes in virus properties, together with the inception of the vaccination campaign on 21 December 2020, underscore the need for continued monitoring of the disease development over time. The present report aims to provide a near real-time description of the characteristics of COVID-19 patients admitted to intensive care units (ICUs) at the onset of a third wave in Switzerland and compare these with the previous course of the COVID-19 pandemic.Methods
The present report is based on the prospective, near real-time observational Risk Stratification in Covid-19 patients in the ICU (RISC-19-ICU) registry, a tool launched on 17 March 2020 to track patient and disease characteristics and the disease course of critically ill COVID-19 patients. The registry is endorsed by the Swiss Society of Intensive Care Medicine (https://www.sgi-ssmi.ch) and was exempt from the need for additional ethics approval and patient informed consent by the ethics committee of the University of Zurich (KEK 2020-00322, ClinicalTrials.gov Identifier: NCT04357275). The study complies with the Declaration of Helsinki, the Guidelines on Good Clinical Practice (GCP-Directive) issued by the European Medicines Agency, as well as the Swiss law and Swiss regulatory authority requirements, and has been designed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for observational studies .Study design and patient selection
The registry contained data from 3344 patients from 69 centres in 14 countries as of 31 May 2021. Given the wide heterogeneity with regard to local viral epidemiology, health care and ICU resources, here we focus only on patients admitted to ICUs in Switzerland. All records as of 31 May 2021 were exported from the registry and divided into two groups. Patients admitted between 1 February and 31 May 2021 were considered to be admitted during a third pandemic wave, based on the increasing number of positive test results for SARS-CoV-2 per 100,000 inhabitants, number of hospitalised COVID-19 patients, and number of COVID-19 patients treated in the ICU in Switzerland, which were observed for the first time in February 2021 following the decline after the peak of the second wave of SARS-CoV-2 infections . Patients admitted between 1 March 2020 and 31 January 2021 were assigned to a comparator group consisting of the patients admitted during the first and second wave of SARS-CoV-2 infections.Registry data collection and transformation
As described in detail elsewhere , a standardised core dataset was prospectively collected during the ongoing COVID-19 pandemic for all critically ill COVID-19 patients admitted to the collaborating centres, and up-to-date cohort characteristics were regularly provided to the Swiss ICUs throughout the pandemic. Inclusion criteria were (i) a laboratory-confirmed SARS-CoV-2 infection diagnosed by nucleic acid amplification according to the testing guidelines issued by the WHO , and (ii) requirement for treatment in an ICU or intermediate care unit, defined as a hospital ward specialised in the care of critically ill patients to provide organ support therapies including invasive mechanical ventilation and/or noninvasive ventilation. Data were collected through an anonymised electronic case report form managed by the REDCap electronic data capture tool hosted on a secure server by the Swiss Society of Intensive Care Medicine . Data were collected on the day of ICU admission and on days one, two, three, five and seven, including patient characteristics, treatment modalities and organ support therapies, the use of mechanical ventilation, vital parameters, arterial blood gas analyses, and laboratory values such as inflammatory, coagulation, renal, liver and cardiac parameters. The Swiss centres enrolled in the RISC-19-ICU represented >60% of all certified secondary versus tertiary care centres in both the North-Eastern and South-Western regions of Switzerland (see supplementary table S1 in the appendix). Registry data transformation for analysis, including the calculation of the disease severity scores Acute Physiology and Chronic Health Evaluation (APACHE II), Simplified Acute Physiology Score (SAPS II) and Sequential Organ Failure Assessment (SOFA) scores, was performed using an openly available code library associated with the registry .Statistical analysis
We report counts and percentages (%), mean and standard deviation (SD) or median (interquartile range [IQR]), as appropriate. The number of positive test results for SARS-CoV-2 infections, number of hospitalised patients and number of vaccine doses applied in Switzerland were provided by the Swiss Federal Office of Public Health (FOPH) . The overall absolute number of patients treated in the ICU was provided by www.icumonitoring.ch / Koordinierter Sanitätsdienst (KSD) and was stratified by age using per-day age distributions derived from the RISC-19-ICU registry. Patient characteristics, physiological status and laboratory measurements at the time of ICU admission were compared between the two groups (first + second vs third wave) using Gaussian (continuous outcomes), logistic (binary outcomes) and Poisson (count outcomes) mixed model analysis . Time period was entered into the model as a fixed effect and treatment centre as random effect. P-values were calculated using a likelihood ratio test of the full model with the effect in question against a “null model” that lacks the effect in question [17], p-values for individual fixed effects were obtained by Satterthwaite approximation . Changes in age distribution throughout both periods were adjusted for the number of comorbidities. Statistical analysis was performed using a fully scripted data management pathway using the R environment for statistical computing version 3.6.3 . Mixed effects modelling was performed using the R-library lme4, version 1.1.21 and graphical output using ggplot2, version 3.2.1 .Source: https://smw.ch/article/doi/smw.2021.20553