Summary
AIMS
With ongoing intensive vaccination programme against COVID-19, numerous cases of adverse reactions occur, some of which represent rare events. Enlargement of the injection site’s draining lymph nodes is increasingly reported, but is not yet widely recognised as being possibly associated with recent vaccination. As patients at risk of a severe course of COVID-19, indicated by their medical history such as a previous diagnosis of malignancy, receive priority vaccination, newly palpable lymph nodes raise concerns of disease progression. In this case series, we report on five patients who presented with enlarged lymph nodes after COVID-19 vaccination.
METHODS
Sonography guided fine needle aspiration (FNA) was performed in five patients presenting with PET-positive and/or enlarged lymph nodes after COVID-19 vaccination with either the Pfizer-BioNTech or Moderna vaccine.
RESULTS
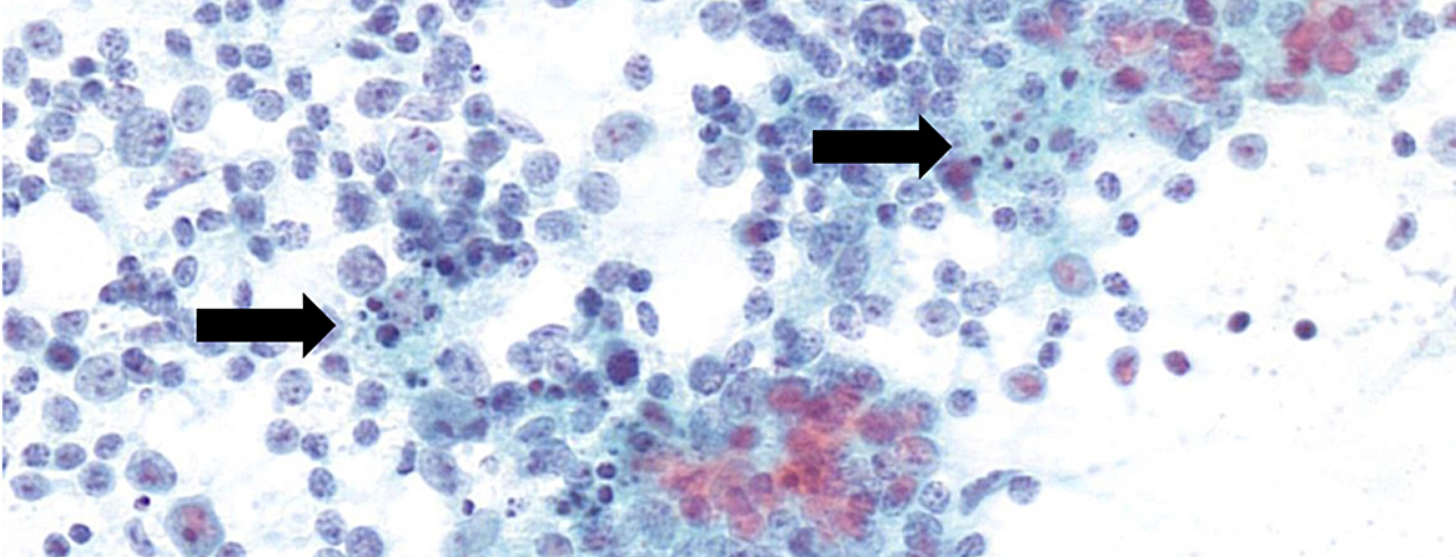
COVID-19 vaccination had been carried out in all cases, with an interval of between 3 and 33 days prior to FNA. Three of five patients had a history of neoplasms. The vaccine was administered into the deltoid muscle, with subsequent enlargement of either the cervical, supra-, infra- or retroclavicular, or axillary lymph nodes, in four out of five cases ipsilaterally. In all cases, cytology and additional analyses showed a reactive lymphadenopathy without any sign of malignancy.
CONCLUSIONS
Evidence of newly enlarged lymph nodes after recent COVID-19 vaccination should be considered reactive in the first instance, occurring owing to stimulation of the immune system. A clinical follow-up according to the patient’s risk profile without further diagnostic measures is justified. In the case of preexisting unilateral cancer, vaccination should be given contralaterally whenever possible. Persistently enlarged lymph nodes should be re-evaluated (2 to) 6 weeks after the second dose, with additional diagnostic tests tailored to the clinical context. Fine needle aspiration is a well established, safe, rapid and cost-effective method to investigate an underlying malignancy, especially metastasis. Recording vaccination history, including date of injection, site and vaccine type, as well as communicating this information to treating physicians of different specialties is paramount for properly handling COVID-19 vaccine-associated lymphadenopathy.
Introduction
COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first registered in China in the end of 2019 from where it rapidly spread all over the globe. The first person in Switzerland was tested positive on 25 February 2020, and the World Health Organization (WHO) declared the disease a pandemic on 11 March 2020 . One year later, more than 170 million cases have been reported to the WHO, with over 3.8 million fatal outcomes . Battling the spread of the virus is dominating public as well as private life, with healthcare institutions constantly challenged by the high workload and management of a novel disease. Researchers have been fervently pursuing the development not only of treatment options, but also of vaccines to provide protection against COVID-19 and eventually end the pandemic .Various vaccines are being evaluated, with currently 322 vaccine candidates and 18 in use worldwide . So far, three have been approved in Switzerland (Comirnaty® by Pfizer-BioNTech on 19 December 2020, COVID-19 Vaccine Moderna® by Moderna on 12 January 2021 and COVID-19 Vaccine Janssen by Johnson&Johnson on 22 March 2021). Over 2500 million doses have been administered globally, with more than 6.7 million in Switzerland alone . As the demand for vaccine delivery is immediate and high, the population was vaccinated in a stratified approach. It is known that COVID-19 infections affect more severely people with certain medical conditions, such as cardiovascular, respiratory, metabolic or immune disorders . Patients with malignancy also carry a considerably higher risk of infection and complications , making it a priority to vaccinate this group. Besides vaccination efficiency, possible adverse events are of concern. Most adverse events following immunisation (AEFI) occur locally and are mild and self-limiting (tenderness at the injection site, reddening, swelling). Systemic AEFI range from fatigue, headache and myalgia to rarely observed severe cases such as anaphylactic shock . Lymphadenopathy may also occur following COVID-19 vaccination and affects mostly ipsilateral cervical, supraclavicular or axillary lymph nodes corresponding to the drainage route after injection into the deltoid muscle. Evaluation of the Moderna COVID-19 vaccine found axillary swelling or tenderness in 11.6% of vaccinated individuals after the first and 16% after the second dose, in 1.1% with clinically detected lymphadenopathy . The Pfizer-BioNTech COVID-19 vaccine trial registered lymphadenopathy in 0.3% of the study participants. This AEFI is most likely underdiagnosed and underreported, leading to potential clinical pitfalls in managing patients who present with a newly palpable mass.
The aforementioned prioritised vaccination of persons with previous or ongoing malignancies represents a further challenge during this COVID-19 pandemic. Newly developed enlargement of lymph nodes raises the question of metastatic disease and elicits diagnostic workup such as imaging or excisional biopsies. Here we describe a case series of five patients referred to the cytology department for a fine needle aspiration (FNA) of enlarged lymph nodes to investigate the presence of neoplastic cells. Our aim is to enhance awareness of COVID-19 vaccine-associated lymphadenopathy and reduce the risk of over-diagnosis as well as overtreatment in suspicious cases. We subsequently propose a set of recommendations on how to approach lymphadenopathy after COVID-19 vaccination.
Material and methods
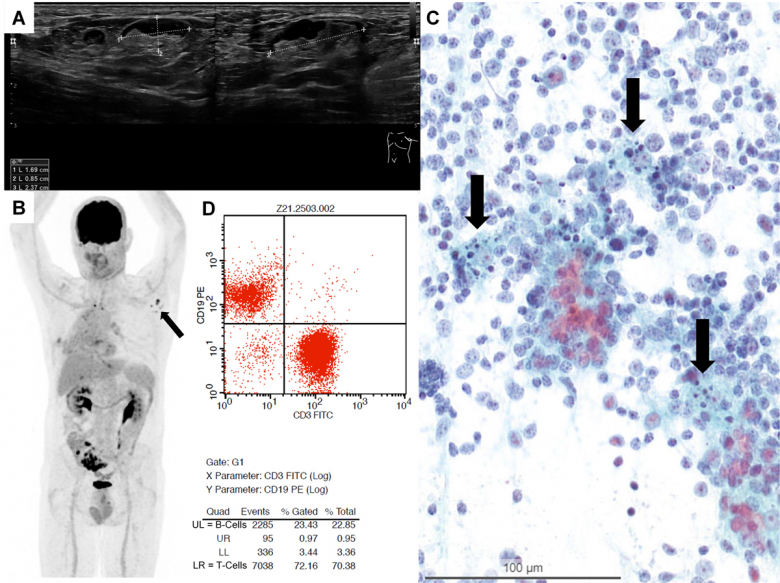
Between 16 February and 3 March 2021, five patients who had recently been vaccinated against COVID-19 with the vaccine by either Pfizer-BioNTech (Comirnaty®) or Moderna (COVID-19 Vaccine Moderna®) were referred for FNA at our institution to evaluate positron emission tomography (PET)-positive or palpably enlarged lymph nodes. Informed consent was obtained for anonymous publishing of diagnostic data in this work. Sonography guided FNA was performed by two experienced cytopathologists (MN, FS). The material was processed and triaged according to standard protocols . Where indicated, additional analysis was initiated. Flow cytometry was performed as described before . Clonality analysis followed standardised guidelines .Results
Patients were referred to our outpatient clinic for FNA of enlarged painless lymph nodes. Two patients (numbers 1 and 5) underwent a PET / computed tomography (CT) scan for follow-up after small cell and adenocarcinoma of the lung. One patient (number 4) was suspected of relapse after incidental diagnosis of a neuroendocrine tumour of the appendix 6 years previously. Two patients (numbers 2 and 3) were female healthcare professionals worried about having metastatic cancer, namely breast cancer, after detecting palpably enlarged lymph nodes on the lower neck, and infraclavicular and axillary sites.All patients presented with palpable or easily detectable superficial lymph nodes on ultrasound in different locations, either in cervical level IV, supra-, infra-, or retroclavicular regions, and in the axilla. Sonographic imaging revealed partially hypo- and partially isoechogenic lymph nodes . The width ranged from 1.0 to 2.4 cm in size, with ovoid to rounded shapes, sharp borders and only partially detectable hilum. Some lymph nodes presented with suspicious sonographic findings (spherical shape with loss of hilum) (see also supplementary fig. S2 in the appendix).
Source: https://smw.ch/article/doi/smw.2021.20557